Changing care. With you.
Working alongside healthcare professionals
We develop solutions that help healthcare professionals take care of their patients and offer associated services that enable them to relieve pain, anaesthetise and improve respiratory functions.
In addition to our focus on chronic care in home healthcare we contribute to take care of patients during acute episodes mainly in the hospital environment.
Our medical gases are also used by healthcare professionals, whether general practitioners or specialists, out of the hospital, and by emergency services.
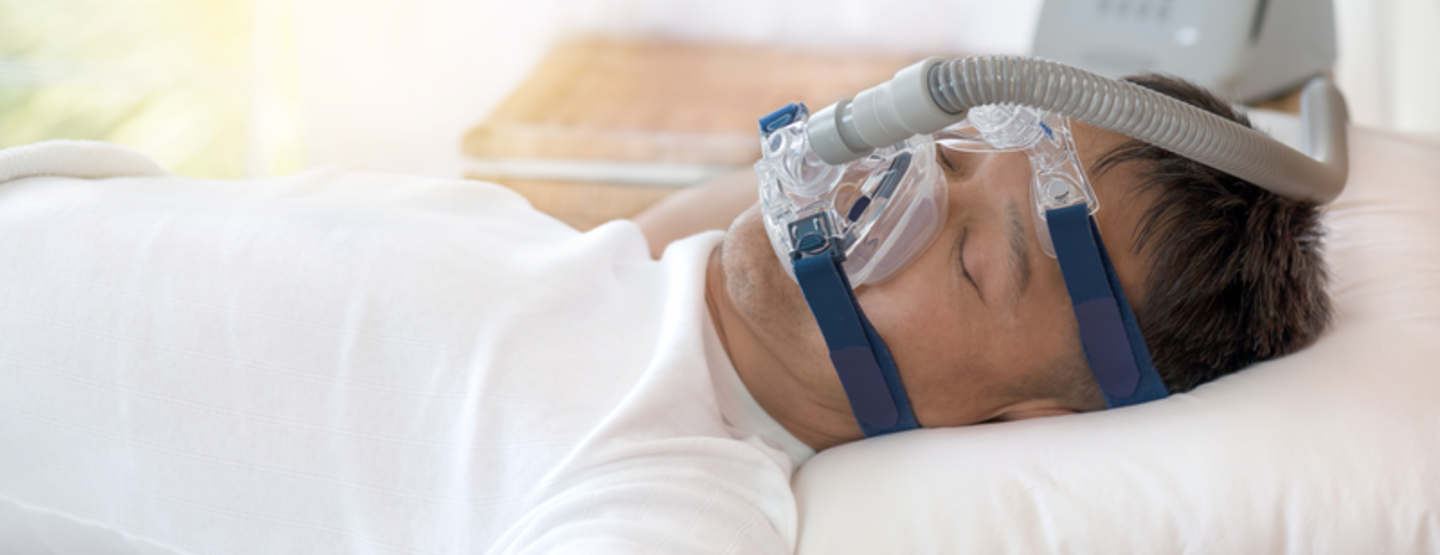
Healthcare products
We are experts in supplying ventilation and respiratory medical equipment for hospitals and home care treatment. Air Liquide Healthcare also manufacture their own range of ventilators and non-invasive ventilation devices, pressure regulators and flow meters for the distribution and administration of medical gases in addition to aerosol therapy devices. For more information on our full range of products including other leading manufacturer's please click below:
Webinar Wednesday - Lunch Time Meeting
Would you like to attend our education and information lunch meeting?
If you have recently started CPAP therapy with us and would like to attend our information evening/afternoon please fill in the application form below so that we can add you to our registration list.
We will contact you via email with the log in details for this invitation only event.
Events will be held last Wednesday of every month (excluding December)
+ 30 years
32 counties
+ 10,000 patients
What's next?
Where to find us?
2 results
-
Blanchardstown -
Dublin
Helpline
Please do not hesitate to contact us if you have any questions.
By telephone
Only urgent calls are accepted outside these hours